Description
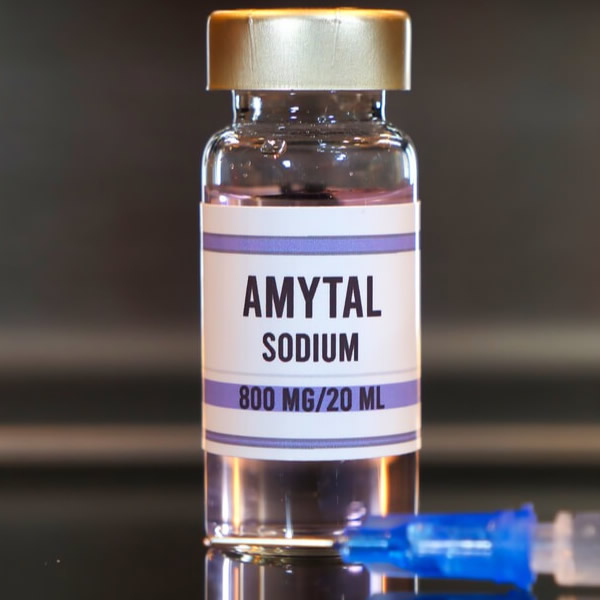
Buy Amytal Sodium (Amobarbital Sodium) Online – Sedative & Hypnotic
Amytal Sodium (Amobarbital Sodium) is a short-acting barbiturate used as a sedative, hypnotic, and preanesthetic agent. It belongs to the central nervous system (CNS) depressant class and is effective in treating insomnia, inducing sleep, and managing acute convulsive episodes.
Key Features of Amytal Sodium
-
Chemical Name: Sodium 5-ethyl-5-isopentylbarbiturate
-
Molecular Formula: C₁₁H₁₇N₂NaO₃
-
Appearance: White, odorless, bitter-tasting powder
-
Administration: Intramuscular (IM) or Intravenous (IV)
-
Maximum Single Dose: 1 gram (adults)
Uses of Amytal Sodium
✔ Short-term insomnia treatment (loses effectiveness after ~2 weeks)
✔ Preoperative sedation
✔ Emergency control of seizures (status epilepticus)
✔ Psychiatric interviews (“truth serum” effect in narcoanalysis)
Dosage & Administration
Intramuscular (IM) Injection
-
Dosage: 65 mg to 500 mg
-
Injection Site: Deep into a large muscle (avoid >5 mL per site)
-
Solution Strength: 20% concentration recommended
Intravenous (IV) Injection
-
For emergencies only (e.g., status epilepticus, severe delirium)
-
Rate: ≤ 50 mg/min (to prevent respiratory depression)
-
Monitoring Required: Blood pressure, respiration, cardiac function
⚠ Warning:
-
High risk of dependence & overdose (controlled substance)
-
Not for long-term use (tolerance develops quickly)
Where to Buy Amytal Sodium Online?
Purchase authentic Amytal Sodium (Amobarbital Sodium) from a trusted online pharmacy:
🔗 Buy Amytal Sodium 500mg Powder (Amobarbital Injection)
Related Products
-
Adderall 30mg (ADHD, Narcolepsy)
-
Ambien 10mg (Insomnia Treatment)
-
Diazepam (Valium) (Anxiety, Muscle Relaxant)
-
Xanax 2mg (Fast-Acting Anxiety Relief)
-
Ketamine Liquid (Anesthetic, Depression Treatment)
Why Choose Us?
✅ Discreet & Secure Shipping
✅ Lab-Tested Pharmaceuticals
✅ Worldwide Delivery
🔞 Legal Disclaimer: Amytal Sodium is a controlled substance. Use only under medical supervision. Misuse can lead to severe health risks or death.
🔗 Shop Now: Buy Amytal Sodium Online




Reviews
There are no reviews yet.